Freeze-drying / Lyophilisation
Process description Freeze dryer and lyophiliser
Freeze-drying or also called lyophilization is a process of extracting water from a moist product by converting it to the solid state and then sublimating the ice. Normally, the wet material, which is in the form of a suspension, solution or wet solid, is frozen at atmospheric pressure. The ice is then converted to water vapor and removed. The remaining dried product is of sponge-like consistency and has approximately the same size and shape as the starting material. It is extremely stable and can easily be restored to its original state with cold water. The aroma and structure are retained, so that in favourable cases there is no difference to the original material. Lyophilization or freeze-drying is used in the production of pharmaceuticals, various foodstuffs, technical products and for restoration.
Freeze-drying takes place in 3 process steps:
1. Freezing
Normally, the starting products to be freeze-dried are complex mixtures of water and other substances. If such substances are cooled below 0°C, pure ice crystals are deposited first. With further cooling, the material becomes rigid and eutectic are formed. Most biological and food products become completely solid at temperatures between -5 and -78°C. After solidification of the entire material, all free water has solidified to ice. The bound water remains fixed within the structure of the substance. Product quality and drying speed depend on the size, shape and size distribution of the ice crystals formed during the freezing process. These properties are also influenced by the homogeneity of the frozen mass. Therefore, the freezing process must be carried out under carefully predetermined and controlled conditions of pressure and temperature and treatment time. During slow freezing, large ice crystals are formed. This can be unfavorable for drying certain substances. On the other hand, freezing too fast causes small ice crystals to form, which can cause undesirable changes in color and structure.
2. Sublimation
For the sublimation step, the frozen substance is exposed to a vacuum. The sublimation of the ice crystals can be divided into the partial steps of heat transfer and mass transfer. Heat is applied to the ice crystals for their sublimation. The water vapor produced is then removed from the surface of the sublimation. This makes it clear that the heat and mass transfer resistances in the material determine the speed of sublimation. The sublimation of 1kg ice requires about 2800kJ. This amount of heat can be transferred by common methods like conduction, radiation, resistance heating, microwaves or infrared radiators. The temperature gradient between the sublimation surface and the heat source is limited by the maximum temperature allowed on the surface of the frozen material. Thus, the dry layer should not be heated up to a temperature at which decomposition begins. When heating by thermal conduction, direct contact of the frozen material with the heating element must be avoided.
3. Desorption
After the sublimation of the ice, further drainage is necessary to remove the bound water that has not crystallized during the freezing process. The temperature is increased and vacuum is applied to remove the bound water.
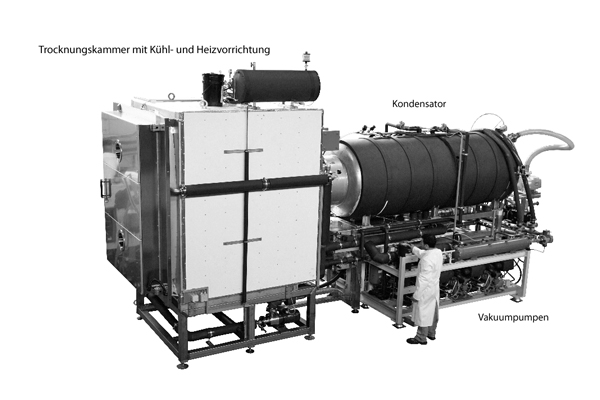
The Freeze Dryer / The Lyophiliser
The freeze dryer or lyophilizator describes the system of the above-mentioned process. From the basic construction, each unit consists of the drying chamber with appropriate heating and cooling elements, a condenser and a vacuum pump. Freeze dryers are usually cooled by a compressor.
Test and contract drying
At ZIRBUS technology you can do test and contract drying on request. We have equipment available at any time to master your drying task and, of course, to develop and produce your customized production freeze dryer or lyophilizator in a pilot plant.
